

The article by Pourcelot et al. (2024) brings a novel approach to wine fermentation. Recently, scientific advances have focused on utilizing microbial consortiums rather than individual species alone or even two individuals co-inoculated. However, spontaneous fermentations are complex, and microbes work in communities. This work aims to design a yeast consortium by studying the population changes over time and determining the metabolite production and fermentation kinetics. In this way, the authors present an elegant molecular approach by tagging each strain to construct a wine fermentation consortium.
References
Eléonore Pourcelot, Audrey Vigna, Thérèse Marlin, Virginie Galeote, Thibault Nidelet (2024) Design of a new model yeast consortium for ecological studies of enological fermentation. bioRxiv, ver.4 peer-reviewed and recommended by PCI Microbiol https://doi.org/10.1101/2024.05.06.592697
The authors have successfully addressed all my previous suggestions. I congratulate them for this nice work, that deserves to be published promptly.
https://doi.org/10.24072/pci.microbiol.100118.rev21
In this revision, authors have incorporated most of the reviewers' comments and as a result the manuscript has improved noticeably. I only have a couple of typos:
L585 fermentation
L595 to have high
L602 expected to have
https://doi.org/10.24072/pci.microbiol.100118.rev23DOI or URL of the preprint: https://doi.org/10.1101/2024.05.06.592697
Version of the preprint: 2
All three reviewers and myself find the manuscript a great contribution to the field. As a recommendation, please address the reviewer's comments.
The manuscript by Pourcelot et al. is a nice and valuable contribution to the growing field of applied microbial ecology, particularly focusing on wine fermentations as a model system. This research is notable for offering a refined model system, which utilizes a set of species tagged with fluorescence for precise population monitoring. It also investigates key research questions, such as how taxonomic diversity influences the performance of ecological communities and how varying environmental conditions may impact this performance.
Major observations:
- The theoretical background supporting the ecological questions addressed in this work could be better elaborated in the introduction, and more importantly, in the discussion of results. The current discussion primarily focuses on previous observations in wine research rather than drawing connections to other studies in theoretical ecology that have addressed similar questions.
- Regarding the two environmental conditions tested (S200 vs. S280), it is unclear whether they genuinely represent contrasting conditions of stress versus non-stress. Does 280 g/L of sugar truly constitute an osmotic stress condition compared to 200 g/L, or does it simply present a more challenging medium for sugar consumption due to cell exhaustion or limited nitrogen availability? While it is evident that osmotic stress persists longer at 280 g/L compared to 200 g/L, it is uncertain if the initial conditions significantly differ in terms of osmotic stress at an ecological or molecular level. If the authors have evidence of stress response induction at 280 g/L versus 200 g/L of sugar, this should be justified in the introduction.
- As the authors propose this consortium as a model system, it would be beneficial to conclude the manuscript (at the end of the discussion) with a clear statement of its strengths and weaknesses. Additionally, outlining the oenological and ecological issues that this model system can address, which previous models could not, would provide valuable context and direction for future research.
Minor comments:
Abstract
Lines 33-34: I suggest to rewrite this sentence not to give the impression that this result is a limitation, as this is an actual and important result. Maybe removing the word “Although” is enough, but, please, consider rephrasing.
Introduction
Lines 68-72: It would be interesting to mention here some relevant works that already used wine microbial consortia to explore fundamental ecological questions (see: https://www.cell.com/action/showPdf?pii=S2405-4712%2817%2930390-3;https://www.nature.com/articles/s42003-023-05284-1;https://www.embopress.org/doi/full/10.15252/msb.202311613)
Material and methods
Line 120: While the assays in mock communities effectively demonstrate that the fluorescent tag accurately indicates yeast cell abundance, it would be beneficial to use qPCR to verify that TDH3 expression remains constant during wine fermentation in monocultures of the six species studied. Perhaps another promoter is needed for Mp.
Line 135: Why was pasteurization chosen over filtration? Did you measure the concentration of residual sugars after pasteurization? Could pasteurization be reducing the actual differences between the S280 and S200 trials?
Line 142: Change "physiological water" to "saline solution."
Line 145: Is there a rationale for using different scales for different trials, or was this simply due to standard laboratory practices and to reduce the cost of the second batch of experiments? If there is a specific reason, please state it here.
Line 171: There is a typo here: change "functionnality" to "functionality."
Line 190: Please revise this sentence. There are some unexpected symbols between numbers and units.
Line 204: please, write “1·105 to 5·105”.
· Lines 212-213: Are there any data available to support this statement?
· Line 234: When stating “They included four CO2 kinetics…,” isn’t it three?
Results
Lines 296-298: This sentence is incomplete.
Line 302: How did you measure and adjust the cell concentration from the pre-inoculum to later test theoretical vs. observed cell counts?
Line 323: If I am interpreting Supplementary Figure S3 correctly, how do the authors explain the detection of cells from other species in monocultures? Are these contaminations or cross-identifications by fluorescence? In addition to the online repository, which is excellent for raw data and scripts, the supplementary material would be better organized in a single file with the corresponding legends to the figures (which have been difficult for this reviewer to find, complicating the interpretation of some results).
· Line 341: When comparing the performance of Sc in monoculture versus as part of consortia, was the concentration of Sc in monoculture 106106? If so, this is 20 times higher than its concentration in the consortia. This should be mentioned to avoid erroneous conclusions about the potential role of the consortia in reducing fermentation kinetics. It is indeed expected that the consortia ferments more slowly compared to S. cerevisiae alone, as the abundance of the primary fermenting agent is reduced.
· Lines 373-375: It is interesting that T. delbrueckii does not consume pyruvic acid (a similar observation is made with Sb, but since Sb is much less fermentative, the observation is less relevant). This should be highlighted as a result.
· Figure 6: It would be beneficial to include a Supplementary Figure to Fig. 6 that shows absolute cell numbers rather than relative abundance. This would allow for a better understanding of whether changes in the relative abundance of a species are due to direct increases or decreases in its absolute cell count, or if they result from changes in other populations while a species remains unaffected by sugar concentration. In this context, did the higher sugar concentration lead to higher maximum cell concentrations of the total consortium and/or individual species? The results should be discussed accordingly.
· Lines 446-447: As mentioned earlier in the "Major comments" section, another possible explanation for this result is that the conditions do not represent significantly different osmotic pressures.
· Line 481: Remove the “)” after “value” at the end of the sentence.
· Lines 479-481: Why is the system described as low complexity? Please explain this more clearly and establish a reference system for comparison (there are examples of both more complex and simpler model systems).
The current manuscript describes the development of a model wine yeast consortium by tagging different yeast species with different fluorescent proteins. The concept is very interesting and relevant to the wine research community. The manuscript is generally well written, results are well presented, and conclusions are sound. I only have minor suggestions for the authors to consider.
L89 check the alpha symbol after DH5, also in L102
L164 saline solution instead of physiological water
L190 check symbols for units
L211-212 it is not clear if this incubation in YPD for 1 hour and then PBS for another hour was the protocol followed by the authors for all samples or only for S. cerevisiae monocultures. Authors should indicate clearly the final protocol followed.
L225 were measured
L271-279 Authors mention in the results section that the phenotype of the transformants was assessed. It would be great if the authors could comment on how many transformants were tested and what were the results.
L279 what is in accordance with previous studies? Please include more information
L298-299 since they…..?
L299 overnight cultures in synthetic grape must (S200)
L318-319 the word fermentation is repeated
L323-328 it would be interesting to see if there is a correlation between the drop in abundance for some non-Saccharomyces and ethanol concentration or dissolved oxygen during the first days of fermentation
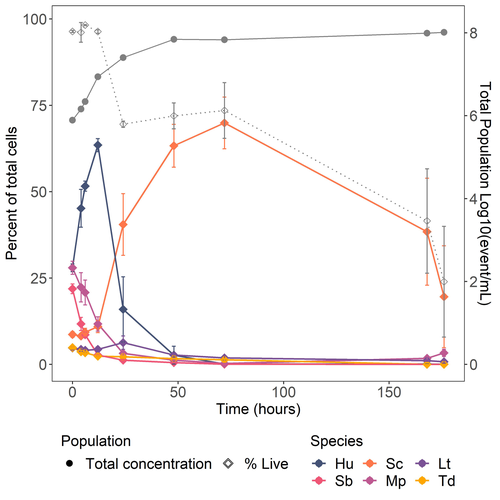
Figure 3 It seems that the symbols in the figures are diamonds while in the legend symbols are circles.
L375-377 please indicate that this sentence refers to pyruvic acid
L469 fluorescent proteins
L500-501 could this be the result of using different S. bacillaris strains?
L510 why is S. cerevisiae the likely cause of yeast viability drop? Please provide more information
L542 ‘to be promoted’ is not the right expression, authors may use ‘dominate’, ‘have a competitive advantage’, etc. also in L546 and L549
L561-563 this sentence is not clear, please check grammar and rewrite
L591 alcoholic
Discussion. In general, we are tempted to think that one particular strain represents the species it belongs to. However, this is not necessarily the case as we know how different S. cerevisiae strains are to each other. This is likely the case with non-Saccharomyces species. I encourage the authors to consider this point and include it in the discussion section.
https://doi.org/10.24072/pci.microbiol.100118.rev13