
Toward a low-energy bioelectrochemical fixation of N2 via mixed cultures electroactive biofilms
 based on reviews by 2 anonymous reviewers
based on reviews by 2 anonymous reviewers
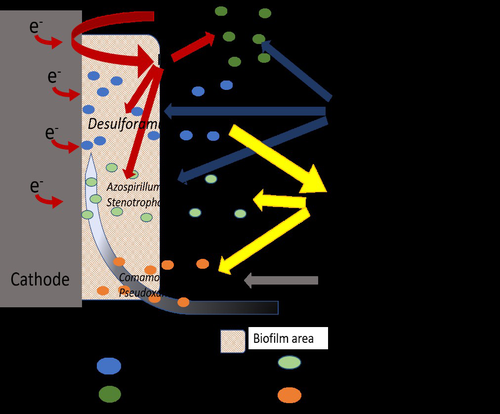
Comparison of enrichment methods for efficient nitrogen fixation on a biocathode
Abstract
Recommendation: posted 07 August 2023, validated 11 August 2023
De Vrieze, J. (2023) Toward a low-energy bioelectrochemical fixation of N2 via mixed cultures electroactive biofilms. Peer Community in Microbiology, 100010. https://doi.org/10.24072/pci.microbiol.100010
Recommendation
Nitrogen fixation and elimination are two key microbial processes that significantly impact the release (and removal) of reactive nitrogen into natural ecosystems. Unlike global change, caused by the emission of greenhouse gasses into our atmosphere, the release of reactive nitrogen into our biosphere only recently (in the last years) received the necessary public attention. Hence, novel techniques for (1) reactive nitrogen recovery, (2) energy-effective removal, and (3) sustainable nitrogen fixation are essential to prevent the nitrogen cycle from spinning out of control without also putting an additional burden on our precious natural resources or increasing the emission of greenhouse gasses.
In this research paper by Rous et al. (2023), the authors investigated the use of a biocathode in a bioelectrochemical system (BES) for sustainable fixation of N2 into NH3, using electricity as a sustainable energy source and CO2 as the only carbon source. A critical element in their study was the enrichment of N2-fixating bacteria, starting from soil samples, in an effort to achieve effective nitrogen fixation. A comparison between the enriched culture and a pure culture of diazotrophic hydrogenotrophic bacteria confirmed comparable results for N2 fixation, indicating that the enrichment process was a viable and successful approach. Although pure culture biotechnological processes have their merits, it is clear that the usage of an enriched microbial culture allows for a more simple, robust, and open microbial process, compared to pure culture systems.
This approach does enable a sustainable way of N2 (and by extension CO2) fixation, as it relies on electricity directly (or indirectly through H2) and CO2 only, but it does suffer from low coulombic efficiencies (<5%). This indicates that, even though the results are promising, there is room for optimization, especially concerning the production of (unwanted) side products, such as acetate and other microbial metabolites. This reflects a key challenge and potential disadvantage of mixed or enriched cultures compared to pure cultures.
It is in that framework that this study provides an interesting, highly relevant view on the potential of bioelectrochemical nitrogen fixation using enriched cultures, yet, it also implies the need to either find a purpose for the byproducts, such as acetate, and/or achieve a more effective enrichment strategy to achieve an increased coulombic efficiency towards sustainable nitrogen fixation.
Reference
Rous A., Santa-Catalina G., Desmond-Le Quéméner E., Eric Trably E. and Nicolas Bernet N. (2023). Comparison of enrichment methods for efficient nitrogen fixation on a biocathode. bioRxiv, 2023.03.02.530809, ver 5, peer-reviewed and recommended by Peer Community in Microbiology. https://doi.org/10.1101/2023.03.02.530809
The recommender in charge of the evaluation of the article and the reviewers declared that they have no conflict of interest (as defined in the code of conduct of PCI) with the authors or with the content of the article. The authors declared that they comply with the PCI rule of having no financial conflicts of interest in relation to the content of the article.
French National Research Agency (ANR, ANR-19-CE43-0013 Cathomix)
Reviewed by anonymous reviewer 1, 20 Jul 2023
The authors have carried out the revisions suggested to them and have also answered the majority of my concerns. The revised manuscript looks much better than the original submission. I have no further comments on the manuscript.
https://doi.org/10.24072/pci.microbiol.100010.rev21Reviewed by anonymous reviewer 2, 28 Jun 2023
The authors have substantially improved the clarity of their manuscript and mostly solved the issue with speculatives assertions. It is now OK for me since some of the data may interrest the community. Yet, I still think the authors could take advantage of all the comments raised by the two reviewers for a future bioelectrochemical study, with a strong focus on: (i) clear sientific questions; (ii) simple yet rigorous methods to answer those questions (i.e. avoiding complex systems with too many putative biotic/abiotic reactions occuring, especially if the latter are not carefully investigated); (iii) sufficiant replication; (iv) performing all the necessary controls. Only this way allows to avoid too speculative statements, which are still too common in the field of microbial electrochemistry.
https://doi.org/10.24072/pci.microbiol.100010.rev22Evaluation round #1
DOI or URL of the preprint: https://doi.org/10.1101/2023.03.02.530809
Version of the preprint: 4
Author's Reply, 22 Jun 2023
Decision by Jo De Vrieze , posted 25 Apr 2023, validated 25 Apr 2023
, posted 25 Apr 2023, validated 25 Apr 2023
Dear Dr. Bernet,
Thank you for submitting your manuscript to PCI Microbiology.
Following the evaluation of your research paper entitled "Comparison of enrichment methods for efficient nitrogen fixation on a biocathode", I have carefully considered the comments of the two highly-qualified reviewers. It is my view and decision that this manuscript has scientific value and that it is relevant for the readers of PCI Microbiology, but Major Revisions are required.
I invite you to resubmit your manuscript after addressing the comments of the two reviewers. When revising your manuscript, please consider all issues mentioned in the reviewers' comments carefully. Please outline in a separate document every change made in response to the reviewers' comments and provide suitable rebuttals for any comments not addressed. Please also not that your revised submission may need to be re-reviewed.
I'm looking forward to receiving your revised manuscript. Please don't hesitate to contact me in case of any further questions.
Best regards,
Prof. Jo De Vrieze
Reviewed by anonymous reviewer 2, 11 Apr 2023
Axel Rous et al. submitted a manuscript entitled “Comparison of enrichment methods for efficient nitrogen fixation on a biocathode”. In this study the authors generate H2 in electrochemical cells which can then be used as electron donor to a mix-community of microbes performing a mix of reactions, including acetogenesis via CO2 reduction and N2 fixation.
This is a very complex system with mixed communities where a lot of microbial metabolisms can occur, and where the latter can interact between each other The fact that the medium in not strictly anaerobic also complexifies interpretations. As such, I found that too many statements were substantially speculative. I would also share my concern over the title and abstract of the manuscript, which somehow struggle to share clear take-home messages with the reader.
Below a list of concerns, questions and remarks:
1) Abstract: “These results confirm the enrichment of autotrophic, electrotrophic and diazotrophic bacteria in the polarized cathode enrichments.” Once there is no more organics, only autotrophic microbes would be expected to possibly grow, isn’t it? So no big surprise there. For “electrotrophic”, the authors bring no proof of such phenomenon (an increase in current density is not enough), and the corresponding discussions are purely speculative.
2) l. 181: you flush the electrochemical cell when there is more than 10% then 5% of O2 in the gas phase. This is very surprising to perform (bio)electrochemical experiments at such low potential
(-0.94 V vs SCE) under the presence of O2. At circumneutral pH, O2 get readily reduced to H2O2 below around -0.3 V vs SCE on conventional carbon electrodes. It would therefore impact the current and generate trace amount of H2O2. The authors should discuss that probable reaction and the corresponding implications.
3) I think Fig. 1 means that there is no nitrogen fixation for nPCE control. However, ammonium production rate for nPCE is the highest in Table 1 (0.07 mg/(L.d) N-NH4, equivalent to 23 mg/L of N-NH4 over the 340 days of the experiment. The authors claim that this NH4 is generated via cell lysis. I am very confused about the fact that a negative control (without supplying electron donor) would generate a product of interest for this study (NH4+) at the highest rate. Could the authors further discuss this result?
4) l.345 Similarly to previous comment: “In nPCE controls, the biomass was higher than in H2 enrichment”.
In Methods, it is stated that the electrolyte used in the electrochemical cells is “the same inorganic medium”, which suggests it was the N-free medium (this should be clear, not suggested). How can biomass grow without any assimilable nitrogen (e.g. ammonium, nitrate) and without ability to fix nitrogen (see Figure 1)? How come it can grow more than the community specifically enriched to fix nitrogen?
5) l. 358: “Regarding the current densities in the abiotic systems, the average current densities were measured at -0.75 A/m2 for two times four days with an organic C source and -1.1 A/m2 for 16 days with only CO2 as the carbon source.” This is unclear, do the author mean they only periodically recorded the abiotic current? This would make this control irrelevant. A rigorous control should be continuously polarized during the whole experiment, which is very long (> 300 d). The cathode has all the time to be modified because of the polarization, for example by reducing trace elements on the carbon surface that would catalyze H2 evolution (or O2 reduction, another issue previously mentioned) and increase abiotic current over time.
6) l. 370: “the current [was] likely due to the direct use of electrons by bacteria in a cathodic biofilm as proposed by Z. Zaybak et al.” At this potential it is common knowledge that (i) H2 evolution occurs; (ii) O2 reduction occurs. The aforementioned statement is therefore extremely speculative. In fully anoxic conditions, the recording of polarization curves could possibly suggest a DET phenomenon if they would exhibit a clear sigmoidal curve that is typical for microbial electrocatalysis. However, the absence of cyclic voltammogram in the manuscript (or SI) makes it impossible to suggest such a microbial electrochemical process.
7) Often, in research related to BES, one would perform basic controls to assess which molecule(s) is involved in the generation of current before making any claim. Here multiple compounds could be related to abiotic and biotic current generation (e.g. reductions of O2, N2, CO2, nitrate and obviously protons to H2). Here I miss relevant tests screening the impact of those different compounds, making any interpretation of the overall electron flow very speculative.
8) Reported coulombic efficiency range from 9% to 63% depending on replicates and period of study. Where are going the other electrons?
9) Communities from one replicate to another can vary to a very large extent, yet here only one “average relative abundance” is provided per enrichment in Fig. 5. I think the Methods section does not provide information on the number of replicates tested. Exhibiting only one result per enrichment would not be very relevant. If several setups were tested for communities, the respective data per setup should be presented.
10) Still about communities, you state (l. 525) that “at the beginning of PCE in bottles fed with organic C, communities were strongly dominated by only four families […] This observation indicated a rapid selection of bacteria having the capability of N2 fixation at the early stage of enrichment.” This is very unclear since you refer to the results expressed either as “at the beginning of enrichment” or “start (in bottle)”. This suggest it is your initial composition of your community, so could it be already “enriched” by a “selection”? If those are not the initial compositions (inocula), where are the latter, since it is obviously necessary to assess an enrichment? Overall the community results are presented in an ambiguous manner.
Some details:
1) What is “H2 enrichment”? Please give clear names to your enrichments and use them consistently along the whole manuscript. Now in Method section you name them “first” and “second enrichment”, then use acronyms or other terminologies: it is confusing. You may also consider that the electrochemical setup in closed circuit would very likely also induce a H2-enrichement at this low cathodic potential, adding to the confusion.
2) l. 117: “The vitamin solution consisted of 0.1g ZnSO4 7H2O, 0.03g MnCl2 4H2O, 0.3g H3BO3 0.2 CoCl2 6H2O, 0.01g CuCl2 2H2O, 0.02g NiCl2 6H2O, and 0.03g Na2MoO4 2H2O per liter of solution.” Those are not vitamins.
3) l. 142: “The monitoring of the current intensity was used to monitor the efficiency in enriching the biofilms in autotrophic and/or electrotrophic bacteria.” How come? At this low potential that can induce abiotic electrochemical reactions, a current alone does not necessary reflect a biological activity.
4) l. 150: “The energy required for the production of microbial metabolites and for biomass growth was then used to calculate the Coulombic efficiency”. Maybe you mean “the electrons required” since there is no thermodynamic data provided for the chemical reactions stated, and obviously there is no energy consideration for assessing a coulombic efficiency.
5) l. 164: “the Coulombic efficiency in percentage of electron recovery in circuit”. The equation above means the opposite: recovery of electrons in products.
6) l. 165: “molproduit”
7) Check the caption of Figure 1, seems there is an issue there.
8) Caption Table 1: “average ammonium”. I guess “production rate” is missing.
9) l. 364: “with regards to the average current in the abiotic controls and standard deviation up to -2 A/m2”. Unclear.
10) l.368: “current consumption”. One do not “consume” current.
11) l. 465: “The C/N ratios are shown in Figure 4. C/N ratios in the PCE were around 10 after 131 and 214 days in comparison with ratio of 5 to less than 1 in the nPCE control. These ratios are consistent with the theoretical ratio of 8 to 10 assumed for microbial biomass.” (i) Please read the sentences yourself to assess the obvious discrepancy between both statements; (ii) for day 214 I read more something like 15 +/- 14.
12) l.468: so what is the other dry carbon coming from?
13) Fig. 4 caption: “Total N concentration based on N measured in dry weight from medium samples for polarized cathode enrichment (PCE) and non-polarized cathode enrichment (nPCE) and estimated from biomass for H2-fed enrichment (H2E)”. Do you dry the medium samples and measure N? Do you express it with respect to liter of sample? Then you only “estimate” it for H2 enrichment medium? I find the caption confusing.
14) l.493: “These high coulombic efficiencies were probably also associated to microaerophilic conditions.” I don’t see why the microaerophilic conditions would increase coulombic efficiency for biomass formation, develop your thoughts. Also, the CE is calculated assuming a constant C/N ratio (Eq. 1) while your results show very different and evolving C/N ratio (Fig. 4,c).
https://doi.org/10.24072/pci.microbiol.100010.rev11Reviewed by anonymous reviewer 1, 09 Apr 2023
This study pertains to studying different approaches for the enrichment of N2-CO2 fixing microbial cultures. Such alternate N2 fixation strategies are highly desired. I have the following queries or comments on the methods and results/discussion sections that should be considered for further improvement of the paper.
· It would be better to provide a clear comparison table for the enrichment methods. This would justify the title of the paper. How these approaches differ from those tested earlier by other researchers should also be stated.
· Line 62: The authors have missed referring to some latest studies on this topic. These include (i) Soundararajan et. al., 2019 (https://doi.org/10.3389/fmicb.2019.01817); (ii) Chen et. al., 2020 (https://doi.org/10.1128/AEM.01998-20); (iii) Yadav et. al., 2022 (https://doi.org/10.1016/j.jcou.2022.101997).
· Line 66-69: It should be noted that not only biomass but some organic compounds can also be synthesized through this approach. For example, Yadav et al. 2022 ((https://doi.org/10.1016/j.jcou.2022.101997) reported on acetic acid production in the N2:CO2 fed bioelectrochemical system.
· Lines 70-98: The generic introduction on microbial N2 fixation can be shortened considerably. Instead, the rationale and objectives of this study should be elaborated further.
· Line 117-118: It is trace metal solution composition and not vitamin solution.
· Line 170: The reasons for applying these different steps in this particular enrichment approach should be stated.
· Line 215-224 – estimation of nitrogen present in biomass –
- This method is not clear.
- For the estimation of actual bacterial count – what all genus were considered?
- Why is theoretical data of E. coli used for estimations?
· Line 243-244 –Why is nifH used as a marker and not any other nif gene? How is this specific N2 fixing activity calculated?
· The calculations for the acetylene reduction assay are missing in the methodology section.
· Lines 345-350: The nifH gene and 16S rDNA gene copies were higher in the nPCE control than H2E experiment. What does it mean as far as the N2 fixation activity is concerned? Were there any other electron donors in the nPCE system?
· Lines 376-379: Any particular reason why there was no revival in the current density performance in spite of having the same experimental conditions after 260 days?
· Table 2: Though PCE 1 and PCE 2 are replicate reactors, a considerable difference in their performance during both periods (particularly the first period) was observed. Why so?
· Table 2 and CE discussion: What are the other possible electron sinks in the PCE reactors? A considerable loss in Coulombic efficiency is seen in both periods (very high in period 2).
· The microbial community discussion part is thorough and appreciated.
Lines 579-580 - How nifH/16s rDNA ratio are compared in terms of percentage? What does the percentage signify here? How can high nifH/16s rDNA ratio be speculated as presence of high N2 fixing bacteria?
https://doi.org/10.24072/pci.microbiol.100010.rev12









