
An important step forward in deciphering coral symbiosis through manipulative approaches
 based on reviews by Tony Robinet and 1 anonymous reviewer
based on reviews by Tony Robinet and 1 anonymous reviewer
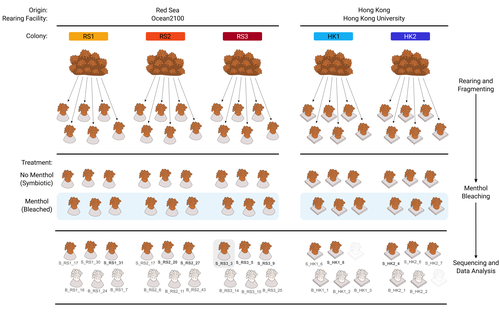
The bacterial microbiome of symbiotic and menthol-bleached polyps of long-term aquarium-reared Galaxea fascicularis
Recommendation: posted 24 May 2024, validated 29 May 2024
Sato, Y. (2024) An important step forward in deciphering coral symbiosis through manipulative approaches. Peer Community in Microbiology, 100048. 10.24072/pci.microbiol.100048
Recommendation
As complex multipartite interactions among the coral host and coral-associated microbial entities including the dinoflagellate symbionts, bacteria, archaea and viruses, have been appreciated, a manipulatable, less-complex study system is desired to deepen our functional understanding of this fascinating symbiotic system. Among experimental manipulation approaches, removal of the algal symbionts using menthol is widely implemented; however, its effect on the rest of the coral-associated symbiotic members has not been explored, which is critical knowledge to assess experimental works using this popular method. This preprint by Puntin et al. (https://doi.org/10.1101/2023.08.23.554380) presents an important observation in this aspect. Their initial observations suggest that menthol-induced coral bleaching introduces stochastic changes in associated bacterial communities, which resemble dysbiosis, making bacterial communities more dissimilar from each other. They also observed low taxonomic diversity in bacterial communities on the corals maintained in aquaria over several months, worth noting as a positive value as an experimental system. Their data are preliminary by nature, while they present intriguing ideas that warrant further studies.
Reference
Puntin G, Wong JCY, Röthig T, Baker DM, Sweet M, Ziegler M (2024). The bacterial microbiome of symbiotic and menthol-bleached polyps of long-term aquarium-reared Galaxea fascicularis (2024). bioRxiv, ver.4., peer-reviewed and recommended by Peer Community In Microbiology. https://doi.org/10.1101/2023.08.23.554380
The recommender in charge of the evaluation of the article and the reviewers declared that they have no conflict of interest (as defined in the code of conduct of PCI) with the authors or with the content of the article. The authors declared that they comply with the PCI rule of having no financial conflicts of interest in relation to the content of the article.
This study is part of the ‘Ocean2100’ global change simulation project of the Colombian-German Center of Excellence in Marine Sciences (CEMarin) funded by the German Academic Exchange Service (DAAD). This study is funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Project number 469364832 (M.Z.) – SPP 2299/Project number 441832482.
Evaluation round #3
DOI or URL of the preprint: https://doi.org/10.1101/2023.08.23.554380
Version of the preprint: 3
Author's Reply, 23 May 2024
Decision by Yui Sato , posted 03 May 2024, validated 03 May 2024
, posted 03 May 2024, validated 03 May 2024
I am in support of reversing the reject decision and would like to authors to submit a slightly revised version before recommendation. Please find my comments in the attached file "Memo on Puntin 2024 MS decision YS.dox.
Download recommender's annotationsEvaluation round #2
DOI or URL of the preprint: https://doi.org/10.1101/2023.08.23.554380
Version of the preprint: 2
Author's Reply, 11 Apr 2024
Dear editorial board of PCI Microbiology,
We are writing to formally appeal the decision to reject the manuscript titled "The bacterial microbiome of symbiotic and menthol-bleached polyps of Galaxea fascicularis in captivity".
We appreciate the time and effort by the reviewers and editors for evaluating our manuscript. We respectfully submit this appeal based on the identification of factual errors and unfair treatment, which collectively had a significant impact on the decision to reject our work.
Please find the detailed explanation in the attached document.
We hope that we can work together to address the concerns raised and contribute valuable insights to the scientific community.
Thank you for your attention to this matter. We look forward to your response.
Best regards,
Giulia Puntin and Maren Ziegler on behalf of the authors
Decision by Cédric Hubas , posted 11 Apr 2024, validated 25 Mar 2024
, posted 11 Apr 2024, validated 25 Mar 2024
First and foremost, I want to extend my sincere appreciation to the authors for their diligent work on this manuscript. The revisions to the experimental protocol have notably enhanced clarity, aligning the message more closely with the results.
However, there remain areas where I diverge from the authors' conclusions. A case in point is the section titled "The microbiome of long-term aquarium-reared Galaxea fascicularis," where the authors propose their hypothesis that captivity leads to the streamlining of the microbiome. This terminology, though technically accurate, may be unnecessarily complex. It essentially refers to the simplification of the microbiome in captive-reared polyps compared to those in the wild. The authors attribute this simplification to the more controlled and stable environmental conditions and the reduced structural complexity of the polyp. While this section also delves into the potential advantages and opportunities presented by this simplified microbiome for experimental manipulation and understanding holobiont functioning, it ventures into discussing the influence of host genotype and environmental conditions on microbiome composition, suggesting avenues for further research to explore these relationships.
However, without a direct comparison of both the microbiome and genomes of the hosts with wild corals, these assertions remain highly speculative.
A reviewer has proposed an alternative hypothesis, which I find more compelling: that the 10-14 days of captivity sufficiently impacted and weakened the bacterial community associated with symbiotic Red Sea polyps to make the effects of bleaching barely perceptible. This idea gains support from the significant presence of a putative coral pathogen (Alteromonas spp.) within the core microbiome of captive Galaxea. Unfortunately, the authors have already dismissed this notion (Line 349), arguing that the reduction or simplification of the microbiome is not an issue associated with captive corals' simplified microbiomes.
Both hypotheses stem from the observation that coral microbiota is simplified in captivity but approach this from different angles. However, without direct comparison data with wild corals and measurements on inputs such as microbiota associated with the corals' diet, etc., it is challenging to ascertain which hypothesis holds greater validity.
Given these circumstances, I anticipate difficulty in crafting a letter of recommendation unless I am personally convinced of the validity of the interpretations drawn from the study's results. I thereby regret to say that despite recognizing the authors' commendable efforts and the significant amount of work they have invested in revising the manuscript, I find myself unable to wholeheartedly recommend the article for publication.
Sincerely,
Cédri Hubas
Reviewed by anonymous reviewer 1, 26 Feb 2024
In the revised ms the authors satisafctorily covered issues raised by the reviewers,
For this reason I believe the manuscript could be accepted
Reviewed by Tony Robinet , 03 Mar 2024
, 03 Mar 2024
Dear authors,
I sincerely acknowledge that changes were made by the authors, mostly in the discussion, and answers brought to my different comments were clear and correct. However it appears that the main correction awaited by the editor ("the inclusion of additional results") was not fulfilled.
During the time since my last reading of this manuscript, I realized a couple of considerations :
1) that Symbiodiniaceae-associated bacteria in coral polypes are triggered by two main parameters : the coral symbionts themselves *and* the surrounding environment. In the Figure S5, the 3 external studies were based on non-bleached corals and clearly confirmed this assumption. In a same way, a majority of the Symbiodiniaceae-associated bacteria present in these external studies were not found in the non-bleached Galaxea ("symbiotic polypes") of the present study.
2) the dispersion of bacterial communities of symbiotic corals from Red Sea in the NMDS (Fig 3) was higher in symbiotic polypes than in bleached polypes (this was not observed for Hong Kong corals). This symptom, a dysbiosis, may be a direct effect of captivity, already affecting symbiotic polypes before bleaching.
Figure 3 and specially the panel B (Bray-Curtis dissimilarities within symbiotic and within bleached colonies), although somehow contradicting this view if not accompanied by statistical tests brouhgt by the authors in the text, was interpreted as indicating "random changes in the communities of the menthol-bleached polyps" (or loss of structure). I agree. In other words, if bleached polyps were dying, the bacterial niche they constituted when Symbiodiniaceae were there continued to destructure and started to collapse. So if the dysbiosis was already there for Red Sea polypes, the bleaching event may just have accentuate an ongoing process. An hypothesis could be that the 10-14 days of captivity has impacted and weakened sufficiently the bacterial community associated with Red Sea symbiotic polypes to make barely perceptible the effects of bleaching.
To my opinion, the weakness in the protocol (effects of captivity couldn't be assessed due to the absence of wild corals and food supply analysis) are not completely insurmountable ; it is not impossible that authors overcome them, by continuing to dig the interpretation of their data and polish the main message.
Experimental microbiology is a steep path, go on and good luck.
Evaluation round #1
DOI or URL of the preprint: https://doi.org/10.1101/2023.08.23.554380
Version of the preprint: 1
Author's Reply, 29 Jan 2024
We express our gratitude to the recommender and reviewers for their assessment of our study and constructive comments, which we have carefully addressed in our authors' reply letter (please refer to the attached PDF).
We have updated the manuscript to incorporate the suggested improvements, involving comprehensive editing to clarify the preliminary nature of our study and underscore its limitations. Specific additions include a new visual summary of the experimental design and analysis, within-colony statistical testing for differences in alpha diversity between symbiotic and bleached polyps (when possible), and a re-plotted Figure S2 displaying raw data points.
We are confident that these adjustments not only align with the constructive feedback received but also improve the quality of our work. We appreciate the time and effort invested in evaluating our manuscript.
With best wishes on behalf of the authors,
Giulia Puntin
Decision by Cédric Hubas , posted 16 Oct 2023, validated 17 Oct 2023
, posted 16 Oct 2023, validated 17 Oct 2023
I trust this letter finds you in good health.
I, along with two different reviewers, have had the opportunity to thoroughly review your study and provide feedback on your submission. Reviewers have articulated several concerns about your preprint, and I wholeheartedly concur with their assessments. The reviewers have highlighted issues such as the relatively low level of replication and the lack of clarity in how measurements were replicated. Additionally, Reviewer 2 has offered valuable insights regarding sequencing and assignment.
The primary concerns raised is about the preliminary nature of the study. While your research presents intriguing insights into the core microbiota associated with the Galaxea fascicularis coral model, the methodology used appears to hinder the generation of clear, conclusive results. The abstract, for instance, implies an expectation of a decisive conclusion regarding the impact of bleaching on the bacterial microbiome of G. fascicularis. However, due to various issues such as the limited number of replicates, sampling from different locations, analysis in different laboratories with slight yet seemingly significant variations in rearing methods, and the absence of comparisons with natural colonies (as pointed out by Reviewer #2), the results come across as over-interpreted. Thus, in my opinion, the study's validity and potential impact must be significantly enhanced with additional results.
After a comprehensive review of your work, I have arrived at the conclusion that I am unable to recommend it for publication in its current state. Below, you will find specific comments that I provide in addition to reviewers comments. Given the methodological limitations, a thorough revision may require the inclusion of additional results.
I cannot make assumptions regarding your ability to supply the requested additional data, so I have opted to request a significant revision rather than an immediate desk rejection. If you are confident in your capacity to furnish additional results and address the queries raised by the reviewers and myself, kindly submit a revised edition of your work. In the event that this proves unfeasible, you will be given the option to withdraw your submission from PCI Microbiology.
Specific comments :
Materials & Methods:
Experimental Design: The experimental design in your study appears to be quite complex, but it is not sufficiently explained. The number of replicates is unclear, which is a critical aspect of any research study. For instance, in the section titled "Sampling for microbial analysis," you mentioned that "n = 15" for both bleached and symbiotic polyps, but it is not clear how this number was determined. Furthermore, the number of polyps collected seems inconsistent with the mentioned number.
Fig. 1: The statement in Fig. 1 that "Alpha diversity remained similar between symbiotic and menthol-bleached samples across all diversity and richness indices tested" is contradicted by the significant difference in Shannon and Simpson indices in RS1 (Red Sea colony #1). This contradiction should be addressed and clarified.
Results:
Fig. 2A and PERMANOVA: In Fig. 2A, the microbial communities from the Red Sea and Hong Kong colonies appear to be very similar, raising doubts about the significance of the PERMANOVA results. It is essential to reconsider this analysis and possibly perform ANOSIM. Considering the clear heterogeneity of multivariate dispersion in Fig 2A, I doubt that betadisper (PERMDISP2) gave a p-value > 0.05.
Discussion:
The main takeout is that bleaching induce a very different response in bacterial communities in « HK » compared to « Red Sea ». This is probably linked to the fact that HK and Red Sea experiments were conducted in different places with slightly different conditions. Unfortunately, it is impossible to test the laboratory/feeding procedure/aquarium effect because this factor has not been replicated which makes it difficult to draw a conclusion. This limitation should be emphasized to provide a more balanced interpretation of your findings.
Conclusion:
The conclusion section in your paper appears to contrast with the abstract. The abstract fails to clearly convey that the difference between bleached and untreated communities is apparently due to stochastic factors. Instead, it suggests "destabilization and loss of structure of the communities," which comes across as vague and overly wordy.
I hope you find these comments helpful in improving the quality of your research.
Sincerely,
Cédric Hubas
Reviewed by anonymous reviewer 1, 02 Oct 2023
In their manuscript, Puntin et al. present very interesting results from their study focusing on the bacterial microbiome of symbiotic and menthol-bleached polyps of Galaxea fascicularis.
However some I have some concerns mosty on the way results are presented.
1. l. 5-6: please rephrase
2. l. 150 (and elsewhere): What is Simpson eveness? I only know Simpson diversity index.
3. Figure 2B: Where is HK1 symbiotic and HK2 bleached in Fig.2B? Apparently you had only two replicates. Did you mention that earlier because I cannot find it.
4. L. 215: You sampled 30 polyps, and excluded 1 from RS3 symbiotic (which one???) due to low seq. depth, so that makes us 29 polyps. Right? In Fig. 2b above you miss two more polyps. You need a table that will show all your samples with proper encoding and will explicitly explain which was used for every analysis. Also you refer to your samples according to origin (i.e. RS: Red Sea) and a number which indicates the colony (1,2,3). However this is confusing since you do not separate the triplicates you sampled from each colony. I would suggest you add a simple encoding (I.e.a,b,c) since it is confusing (e.g. which RS3 sample was excluded????).
Reviewed by Tony Robinet , 25 Sep 2023
, 25 Sep 2023
Comments on the MS from Giulia et al. sent to PCI microbiol
################################################
The authors aimed at evaluating the behaviour of microbial community in the tropical coral Galaxea fascicularis after polyps from the same wild colonies were kept in captivity under controlled conditions only, or under controlled conditions and bleached with menthol.
Bleaching, corresponding to the disappearance of the photosymbiontic Symbiodiniaceae from the polypes, induced a disorganisation in microbiomes in the way that the structure formed by core taxa in symbiotic polypes vanished, turning into a kind of stochastic assemblage of taxa. Authors did not notice any typical signature of bleaching, like would have been the systematic death of some key-taxa.
Authors discussed that, in this study, captivity did reduce the diversity of microbiomes in polypes, compared to those living in non-captive ones, but there were no assessment of wild polypes microbiomes in this study. The comparison relied on data from literature only. However, the captivity effect, i.e. the lack of exogenous wild bacterial flow into bleached polypes, and the potential bacterial flow form food, were appropriately proposed to explain the observed convergence of microbiomes of Red Sea and Hong Kong due to their captivity in similar conditions.
Results are presented by a scientific team who is experienced in coral microbiology, as we can read it in introduction and discussion. Concepts are well defined, literature is recent and abundant, questions are clearly addressed, scripts are clean and working.
A complete study of the bleaching effect would probably have gathered more samples (here only 14 from 5 colonies), sequenced "wild" samples in coral colonies of the same locations where captive ones have been collected, developped a correct sequencing protocol for the Symbiodiniaceae ITS2 (this axis is unfortunately under-explored), and analyzed the unknown microbial contamination brought by feeding (L103: "polyps were fed daily with one small frozen adult Artemia each"). We can understand all the reasons explaining why these elements are lacking, but in their absence, I think that this study can be worth to be shared with the scientific community if authors present their results as preliminary, or with these gaps expressed in the abstract, before a complete study can be lead. As well, the title should be clarified by mentioning the fact that "symbiotic" and "bleached" corals were both captive.
I have no specific comments, the manuscript is well written, only a specific question : Why did you assigned taxa only to genus rank, and then numbered the ASV ? (= the unique sequences, i.e. all variants found on this marker in each species), given that (1) Silva database assignation is quite good down to species rank ; (2) if assignation with qiime is not robust for a given taxa, species is named "unassigned species" ; (3) 16S marker is known to be prone to an unknown number of copies in a same organism, with possible nucleotidic variation between copies, and therefore with the possibility to over-estimate the effective number of different organisms, and thus the reality of some of them ? Or maybe you know that Symbiodiniaceae have only one 16S copy ? Did you try the same analyses at the species level (97% of similarity between sequences)?










